Are you interested in finding 'luminol synthesis'? You will find the answers here.
Luminol is synthesized aside the dehydration chemical reaction of 3-nitrophthalic bitter with HydrazineHydrazine is an inorganic trifoliated with the chemic formula N2H4. Information technology is a plain pnictogen hydride, and is a dull and flammable graceful with an ammonia-like odor. Hydrazine is highly toxic and dangerously unstable unless handled in solvent as , hydrazine hydrate. As of 2015, th…. The reaction is hot to remove body of water, and triethylene dihydric alcohol is added to further increase the temperature. The nitro group of the 3-nitrophthalhydrazide is past reduced using Na dithionite to class an amino grouping at high pH.
Table of contents
- Luminol synthesis in 2021
- Synthesis of luminol pre-lab
- Luminol experiment
- Luminol and hydrogen peroxide reaction
- Synthesis of luminol lab report
- Luminol reaction
- Luminol synthesis mechanism
- Luminol chemiluminescence lab
Luminol synthesis in 2021
 This image illustrates luminol synthesis.
This image illustrates luminol synthesis.
Synthesis of luminol pre-lab
 This image illustrates Synthesis of luminol pre-lab.
This image illustrates Synthesis of luminol pre-lab.
Luminol experiment
 This image illustrates Luminol experiment.
This image illustrates Luminol experiment.
Luminol and hydrogen peroxide reaction
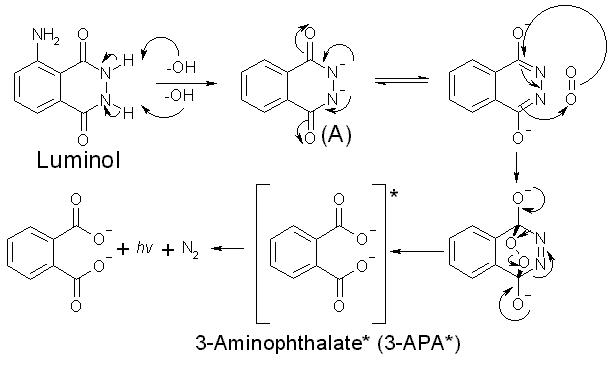 This image representes Luminol and hydrogen peroxide reaction.
This image representes Luminol and hydrogen peroxide reaction.
Synthesis of luminol lab report
 This image demonstrates Synthesis of luminol lab report.
This image demonstrates Synthesis of luminol lab report.
Luminol reaction
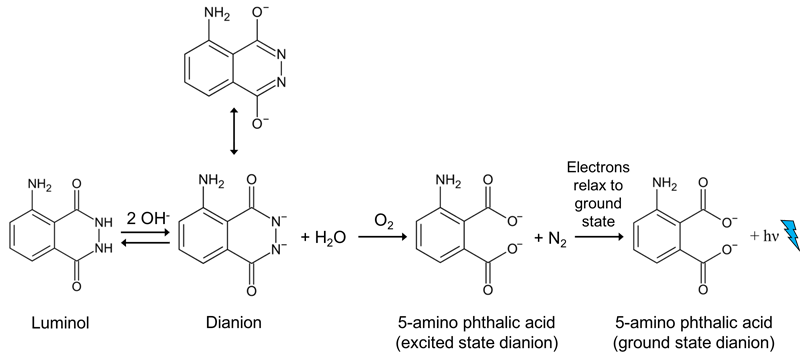 This picture illustrates Luminol reaction.
This picture illustrates Luminol reaction.
Luminol synthesis mechanism
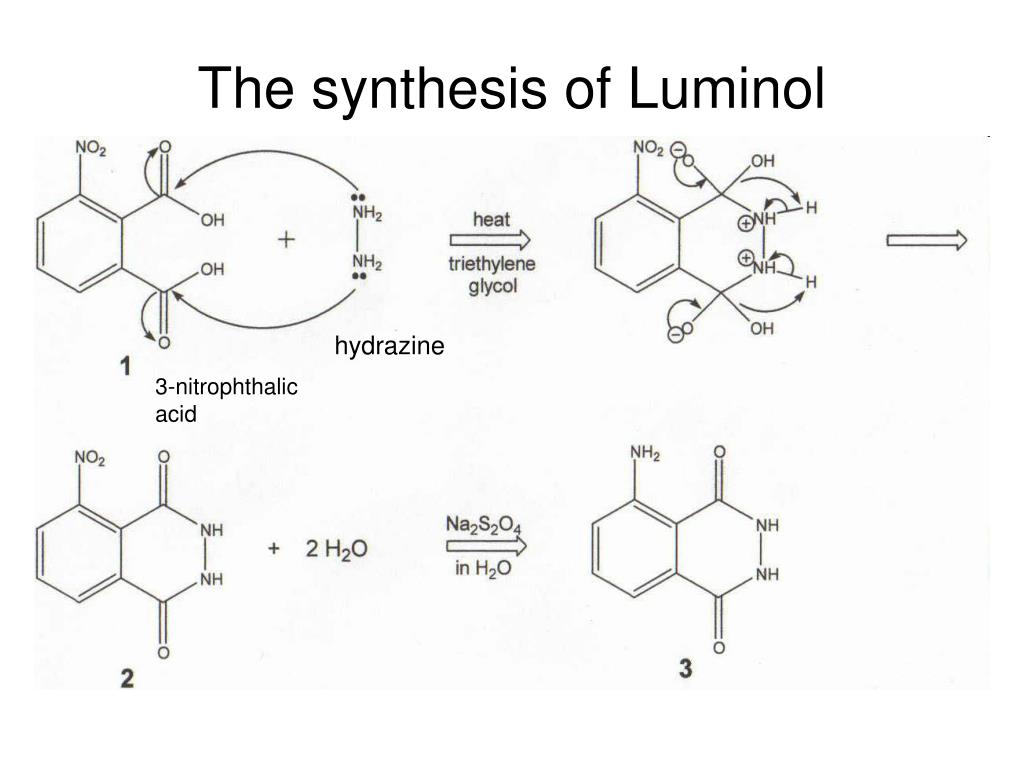 This picture representes Luminol synthesis mechanism.
This picture representes Luminol synthesis mechanism.
Luminol chemiluminescence lab
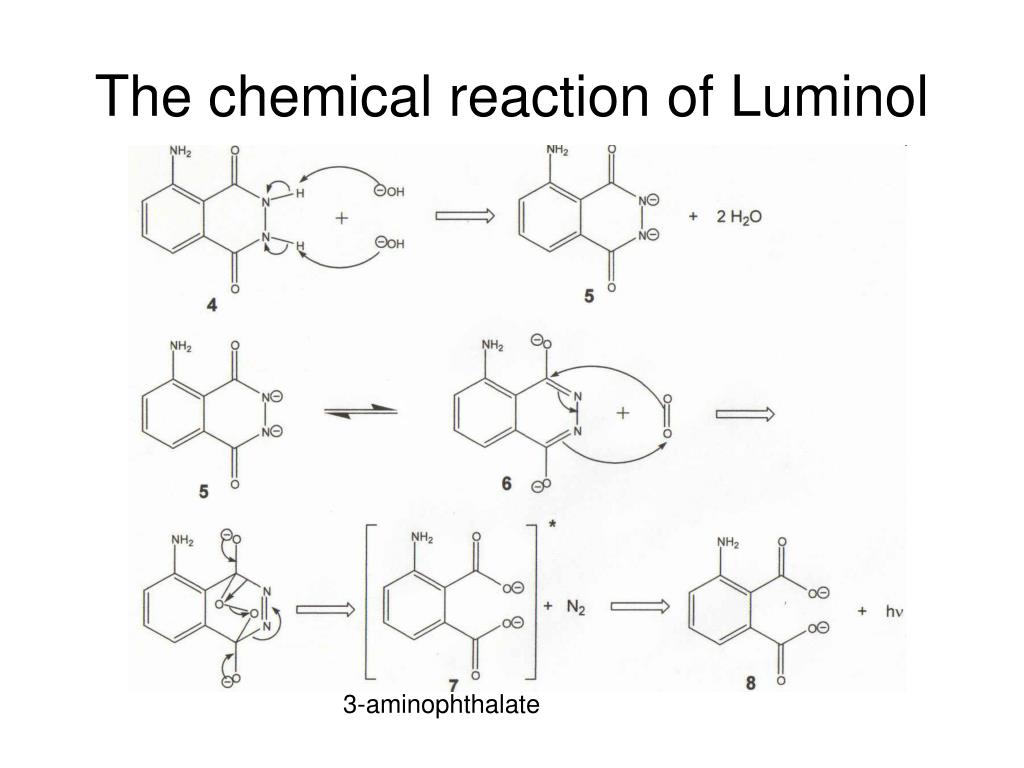 This picture representes Luminol chemiluminescence lab.
This picture representes Luminol chemiluminescence lab.
How is luminol used to detect trace amounts of blood?
The investigator sprays a solution of luminol and the oxidant. The iron in blood catalyses the luminescence. The amount of catalyst necessary to cause the reaction is very small relative to the amount of luminol, allowing detection of even trace amounts of blood.
What happens when Luminol is oxidized to anionic radical?
Luminol is first deprotonated in basic conditions, then oxidized to the anionic radical. Which in turn has two paths available to give the key intermediate α-hydroxy- peroxide. After cyclization to the endoperoxide, the mono-anion will undergo decomposition without luminescence, if the pH is too low (< 8.2) for a second deprotonation.
Which is the product of chemiluminescence of luminol?
Chemiluminescence. The oxygen produced from the hydrogen peroxide then reacts with the luminol dianion. The product of this reaction—an unstable organic peroxide —is made by the loss of a nitrogen molecule, the change of electrons from excited state to ground state, and the emission of energy as a photon. This emission produces the blue glow.
How long does it take for luminol to make a blue glow?
When luminol is sprayed evenly across an area, trace amounts of an activating oxidant make the luminol emit a blue glow that can be seen in a darkened room. The glow only lasts about 30 seconds, but can be documented photographically.
Last Update: Oct 2021