Are you searching for 'recrystallization of benzoic acid outline essay'? You can find questions and answers on the topic here.
Table of contents
- Recrystallization of benzoic acid outline essay in 2021
- Recrystallization of acetanilide lab report
- Impure benzoic acid
- Organic chemistry recrystallization lab report
- Benzoic acid melting point
- Recrystallization
- Recrystallization lab report conclusion
- Recrystallization of benzoic acid outline essay 08
Recrystallization of benzoic acid outline essay in 2021
 This image demonstrates recrystallization of benzoic acid outline essay.
This image demonstrates recrystallization of benzoic acid outline essay.
Recrystallization of acetanilide lab report
 This picture representes Recrystallization of acetanilide lab report.
This picture representes Recrystallization of acetanilide lab report.
Impure benzoic acid
 This image illustrates Impure benzoic acid.
This image illustrates Impure benzoic acid.
Organic chemistry recrystallization lab report
 This picture demonstrates Organic chemistry recrystallization lab report.
This picture demonstrates Organic chemistry recrystallization lab report.
Benzoic acid melting point
 This image representes Benzoic acid melting point.
This image representes Benzoic acid melting point.
Recrystallization
 This picture shows Recrystallization.
This picture shows Recrystallization.
Recrystallization lab report conclusion
 This image illustrates Recrystallization lab report conclusion.
This image illustrates Recrystallization lab report conclusion.
Recrystallization of benzoic acid outline essay 08
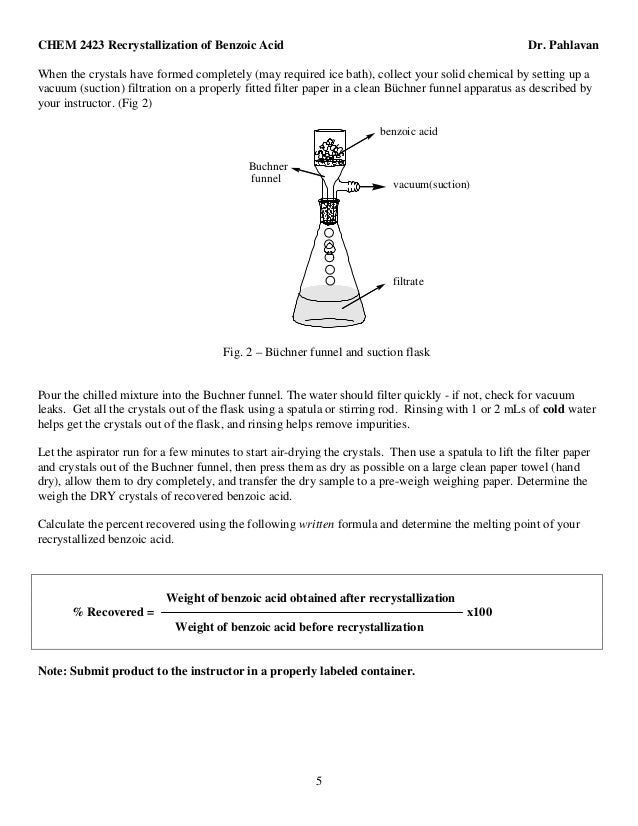 This image representes Recrystallization of benzoic acid outline essay 08.
This image representes Recrystallization of benzoic acid outline essay 08.
What is the objective of recrystallization of benzoic acid?
Recrystallization of Benzoic Acid Objective To purify benzoic acid by recrystallization and gain experience with a basic organic laboratory techniques. Background Products of chemical reactions are often contaminated with impurities.
When to write an experimental report on benzoic acid?
Experimental Report Write an “experimental” describing the procedure that you followed to recrystallize the benzoic acid. A first draft of the experimental is due one week after steps 1–10 have been completed. A final copy that includes the final mass and the melting point of the benzoic acid is due one week after step 11 is completed.
How is recrystallization used to purify chemical reactions?
Background Products of chemical reactions are often contaminated with impurities. One method for purifying chemicals, recrystallization, takes advantage of the differences in the solubilities of the desired products and the impurities and the tendency for the slow formation of crystals to exclude impurities from the crystalline solid.
How can benzoic acid be separated from an ionic solid?
From the balanced chemical equation, it is clear that the benzoic acid will not be pure since the byproduct MgClBr, an ionic solid, forms at the same time the desired product forms. Benzoic acid can be separated from ionic solids because the materials have different solubilities in water.
Last Update: Oct 2021